PBMCs
Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs (Peripheral Blood Mononuclear Cells) constitute a fraction of the peripheral blood leukocytes including T and B lymphocytes, NK cells, monocytes and dendritic cells. PBMCs are often used as starting material to isolate specific cell subset to be used in different assays.
How are PBMCs prepared and characterized?
PBMCs are prepared from Leukopaks through density gradient separation and cryopreserved after performing cell count.
PBMCs are filled in excess to account fo possible cell loss during the cryopreservation process and to guarantee the minimum of viable cell counts indicated in the specifications. The frozen PBMCs are later thawed to perform quality control and viability and cell count are confirmed. BIOMEX isolates PBMCs from Leukopaks isolated in the own plasma centers and can guarantee cryopreservation within 2h from withdrawal, resulting in high quality, unstimulated PBMCs available in large lots for optimal consistency across multiple experiments.
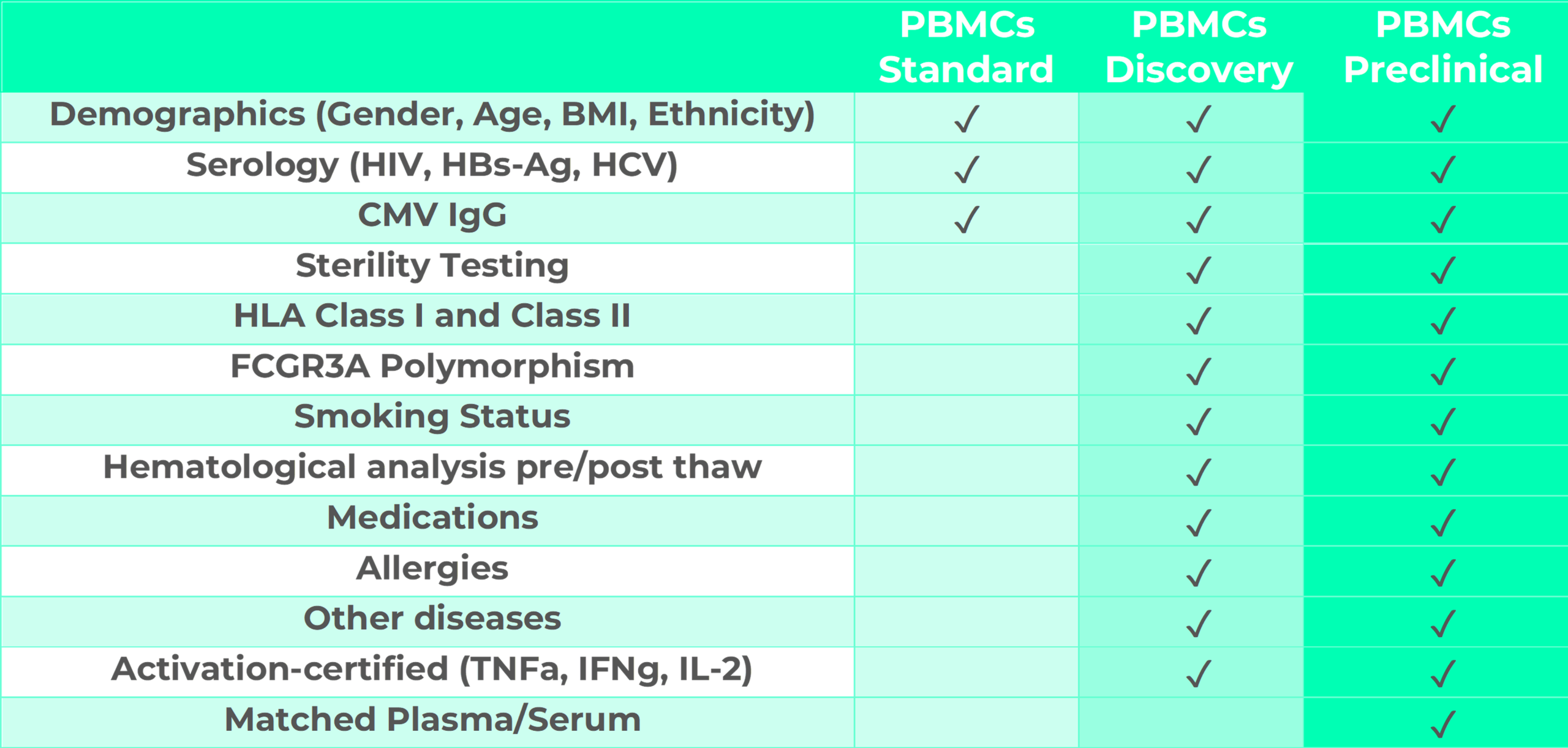
Every PBMCs lot is tested for sterility and cell activation upon stimulation. Moreover, PBMCs come with donor information including demographics, serology, HLA class I and II characterization, FCGR3A Polymorphisms, smoking status, hematological analysis pre- and post-thaw, medications, allergies and eventual other diseases. Data are made available according to the chosen plan.

What are typical PBMCs applications?
PBMCs play a major role in the study fields of immunology, immuno-oncology and in the development of Cell and Gene Therapies.
Particular valuable information can be derived from the choice of donors and donor combinations presenting suitable MHC Class I or Class II. MHC (Major Histocompatibility complex), also called HLA in humans (Human Leukocyte Antigen) are a set of genes coding for cell surface proteins essential for the recognition of foreign molecules by the immune system. HLA Class I are expressed by every human cell and present peptides produced by processing the proteins inside the cell. These peptides are recognized by CD8+ T-cytotoxic cells, which screen for sequences expressed by intracellular pathogens and eliminate the infected cells. HLA Class II are expressed by particular cells called Antigen Presenting Cells (APC), which are responsible for detecting foreign proteins outside the cells and for presenting them to the rest of the immune system in order to start an immune reaction.
The study of HLAs constitutes a critical aspect of donor/recipient matching in bone marrow transplantation and plays a major role in cancer vaccine development, in the study of on-and off-target toxicity of immune-based cell and gene therapies, in the immunogenicity testing for large molecules and in patient stratification for drug discovery in the immuno-oncology field.
PBMCs with HLA details are therefore often used:
- In the development of therapeutic antibodies such as TCR-like antibodies (Targeting the peptide MHC, also pMHC antibodies)
- In the study of immunogenicity of Large Molecules, particularly the development of anti-drug antibodies (ADAs)
- In the study of immunotoxicity linked to cross-reactivity of therapeutic antibodies on multiple tissues (causing cytokine storm or direct cytotoxicity)
- In the development of anti-cancer vaccines as therapeutics
- In the development of TCRs for the selection of HLA-TCR-Peptide complexes with high immunogenicity.
- In tumor infiltration studies and in the study of the interaction between tumor and the immune system
- In inflammation and wound repair studies
- In HLA class I –matched co-culture studies with other cell types in order to reduce background cell activation.
BIOMEX‘s PBMCs plans
This plan is designed for basic research and is ideal for preliminary studies or assays which do not require advanced donor-related information. PBMCs-Budget cells are tested for serology. Additional optional information and tests can be provided against a small fee as presented in the plan below.
This plan is designed for assays to be employed in drug discovery approaches including target validation, lead identification and optimization. It provides complete donor information including demographics, serology, HLA class I and II characterization, FCGR3A Polymorphisms, smoking status, hematological analysis pre- and post-thaw, medications, allergies and eventual other diseases. PBMCs-Discovery are tested for sterility and cell activation.
This plan is designed for assays to be employed in preclinical assays including immunogenicity, immunotoxicity, mechanistic toxicology and investigative toxicology. PBMCs-Preclinical provided with complete donor information including demographics, serology, HLA class I and II characterization, FCGR3A Polymorphisms, smoking status, hematological analysis pre- and post-thaw, medications, allergies and eventual other diseases. PBMCs-Discovery are tested for sterility and cell activation. In addition, matched plasma or serum are available from the same patient in 1ml cryopreserved aliquots.
Our PBMCs offering includes the ImmunoSafe™-HLA1 and ImmunoSafe™-HLA2 panels for immunogenicity and immunotoxicity testing with or without matched plasma and serum.
Our Experts for Life Sciences
Unlock the potential of your research with our high-quality biospecimens and expert services. At BIOMEX, we believe that working with our customers is the best way to improve project success and reduce costs and development time.


