Cellular Starting Materials
Primary Cells for Cell and Gene Therapy
Fresh and cryopreserved primary cells are used for the discovery and process development of Cell and Gene Therapies, either as a target of the therapy in efficacy and potency studies, as immune assays in safety studies or as starting material for process development, manufacturing and training.
Regardless of your development stage: design your experiments with the science in mind, partner with BIOMEX to bring them to life!
Find out how BIOMEX can support you bringing your Therapy from discovery to the clinic, reduce time to market and de-risk your pipeline.
Materials for Cell and Gene Therapy discovery and process development
Donor variability, stability of supply, workflow flexibility and quality of the biological materials are among the main concerns for Cell and Gene Therapy developers during the discovery and process development stages. BIOMEX sources and delivers its products from its own plasma centers and manufacturing facilities in central Europe, maintaining control of its quality standards on the whole supply chain from isolation to delivery.
While fresh materials are often employed during the late discovery and process development phases as they most closely resemble the real-life scenario in the clinic, cryopreserved cells can serve as optimal starting point for early discovery, enabling more flexibility and reproducibility in experimental design in smaller scales. Similarly, cryopreserved Leukopaks enable more predictability in process development by uncoupling experimental plans from the logistics of fresh materials.
Click here and find out more about:
Fresh Leukopaks
Fresh Leukopaks are the result of a cell concentration process starting from healthy and consenting donors’ blood (leukapheresis) according to an IRB-approved protocol. Fresh Leukopaks can be used as a starting material for the isolation of specific immune cell types to be cryopreserved and employed in the discovery phase or as a starting material for process development. The concentration of leukocytes in Leukapheresis is higher than in whole blood, and the concentration of mononuclear cells (MNCs) is higher than in buffy coats (typical concentration up to 20% monocytes, 50% T-Cells, 10% B-Cells, 10% Natural Killer cells, 3% granulocytes).
Isolating individual cell types from Leukopaks allows to achieve high cell yields and reduce the donor-dependent variability between experiments. It is nonetheless important to consider that individual donor characteristics such as age, height, gender, BMI, smoking habits or a particular clinical history can result in variability in assay performance and in the proportion between the cellular components. Increasing donor variability in your studies can improve process robustness in the latest development stages and even gain important insights in outliers. If you are interested to integrate more donor variability in your safety studies, check out our Immunogenicity – Immunotoxicity page, or contact us.
Fresh Leukopaks are filled to yield a minimum of 10 billion (whole Leukopak) or 5 Billion (half Leukopak) at release. However, we chose to prioritize speed of delivery and quality of the product over absolute cell count, and deviations >5% on the total cell count comparing to the indicated minimum will be granted as automatic discount on one bag of the following order.
Fresh Leukopaks are directly isolated and delivered by BIOMEX in its own plasma centers in Germany and can be delivered within 24h from isolation in the whole Central Europe. Upon isolation you will receive a tracking link with the information to access protocols, certificate of analysis and other relevant details. Parameters reported on the certificate of analysis include demographic data and donor information, HLA-Typing, Complete Blood Count and pathogen testing at donor level. Additional tests can be added on request.
To inquire about individual lead times for individual donor specifications, the possibility to recall a particular donor or for delivery to countries outside Central Europe, please contact our sales team.
Cryopreserved Leukopaks
Cryopreserved Leukopaks are the result of a cell concentration process starting from healthy and consenting donors’ blood (Leukapheresis) according to an IRB-approved protocol, followed by a controlled-rate cryopreservation step. Cryopreserved Leukopaks can be used as a starting material for the isolation of specific immune cell types to be further cryopreserved and employed in the discovery phase or as a starting material for process development. In this respect, Cryopreserved Leukopaks are particularly suitable for process development, as they enable to uncouple the experimental plan from the logistics of the raw material, allowing you to start your experiment whenever you are ready and save important time and resources during the process.
The concentration of leukocytes in Leukapheresis is higher than in whole blood, and the concentration of mononuclear cells (MNCs) is higher than in buffy coats (typical concentration up to 20% monocytes, 50% T-Cells, 10% B-Cells, 10% Natural Killer cells, 3% granulocytes). Cell viability and the cell count of the different cell population are similar between fresh and cryopreserved Leukopaks and reported on the certificate of analysis.
Parameters reported on the certificate of analysis include demographic data and donor information, HLA-Typing, Complete Blood Count and pathogen testing at donor level. Additional tests can be added on request for new productions.
To inquire about individual lead times for individual donor specifications, the possibility to recall a particular donor or for delivery to countries outside Central Europe, please contact us.
PBMCs
PBMCs (Peripheral Blood Mononuclear cells) constitute a fraction of the peripheral blood leukocytes including T and B lymphocytes, NK cells, monocytes and dendritic cells. PBMCs are often used as starting material to isolate specific cell subset to be used in different assays.
PBMCs are prepared from Leukopaks through density gradient separation and cryopreserved after performing cell count. PBMCs are filled in excess to account fo possible cell loss during the cryopreservation process and to guarantee the minimum of viable cell counts indicated in the specifications. The frozen PBMCs are later thawed to perform quality control and viability and cell count are confirmed. BIOMEX isolates PBMCs from Leukopaks isolated in the own plasma centers and can guarantee cryopreservation within 2h from withdrawal, resulting in high quality, unstimulated PBMCs available in large lots for optimal consistency across multiple experiments.
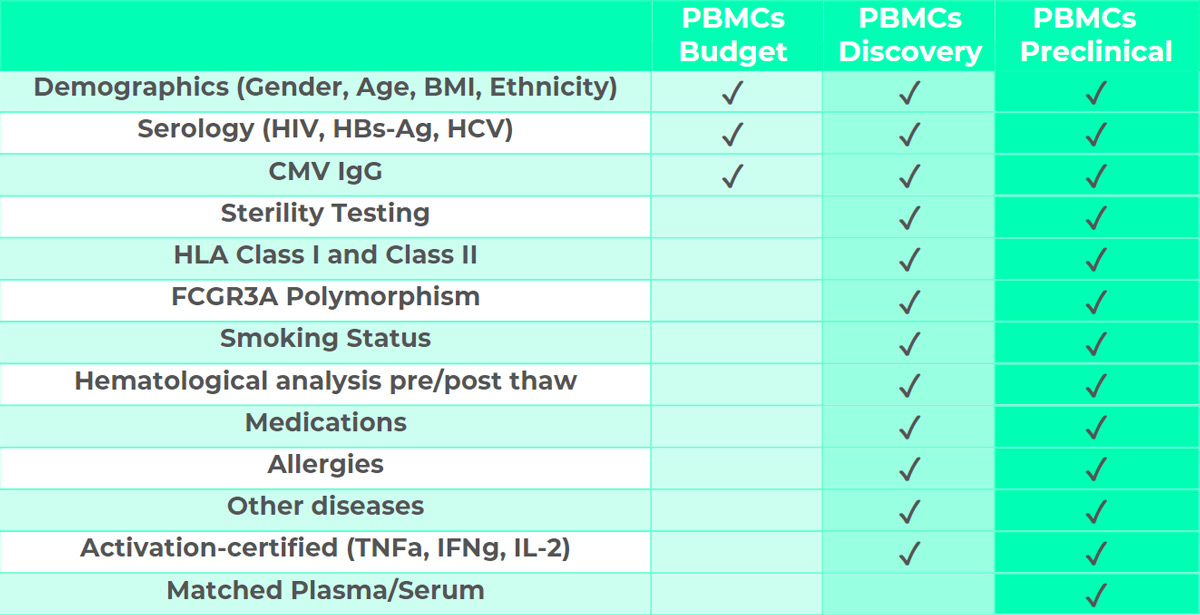
BIOMEX‘s PBMCs plans
This plan is designed for basic research and is ideal for preliminary studies or assays which do not require advanced donor-related information. PBMCs-Budget cells are tested for serology. Additional optional information and tests can be provided against a small fee as presented in the plan below.
This plan is designed for assays to be employed in drug discovery approaches including target validation, lead identification and optimization. It provides complete donor information including demographics, serology, HLA class I and II characterization, FCGR3A Polymorphisms, smoking status, hematological analysis pre- and post-thaw, medications, allergies and eventual other diseases. PBMCs-Discovery are tested for sterility and cell activation.
This plan is designed for assays to be employed in preclinical assays including immunogenicity, immunotoxicity, mechanistic toxicology and investigative toxicology. PBMCs-Preclinical provided with complete donor information including demographics, serology, HLA class I and II characterization, FCGR3A Polymorphisms, smoking status, hematological analysis pre- and post-thaw, medications, allergies and eventual other diseases. PBMCs-Discovery are tested for sterility and cell activation. In addition, matched plasma or serum are available from the same patient in 1ml cryopreserved aliquots.
Our offering for Cell and Gene Therapy safety testing
Safety testing for Cell and Gene Therapies constitutes one of the most important steps during preclinical and clinical development. While adoptive cell therapies (ACTs) including T-cell receptor engineered T cells (TCR-T), chimeric antigen receptor T-cells (CAR-T) and tumor-infiltrating lymphocytes (TIL) have proven to be greatly effective for cancer treatment, they have been associated with mild to life-threatening on-target toxicity, off-target toxicity and toxicity resulting from immune activation such as the Cytokine Release Syndrome (CRS). Similarly, Gene Therapies have been reported to be associated to on- and off-target toxicity. Moreover, especially for adeno-associated viruses (AAV), the high doses of viral vector necessary to overcome the baseline production of antibodies directed against the capside proteins had led to serious adverse events such as hepatotoxicity and dorsal root ganglia toxicity.
Our offering for Cell and Gene Therapy safety testing includes PBMCs panels for immunogenicity and immunotoxicity testing with or without matched plasma and serum. If you are looking forward to saving time and resources, our expert Service Team will partner with you to accelerate your pipeline progress. For further information, please contact us.
Immunogenicity and Immunotoxicity panels
BIOMEX’s Immunogenicity and Immunotoxicity panels are designed to support the in-house safety testing of Large Molecules, Cell Therapies and Gene Therapies during the preclinical and clinical phases. The panels are assembled in a configuration suitable to reproduce the whole range of the most frequent HLA class I or class II alleles in the Caucasian population and are suitable for the the first screening assays. Panels to perform more specific assays with particular alleles can be assembled upon request.
The Immunogenicity and Immunotoxicity panels include each 100 vials, corresponding to 10 vials à 20 Mio viable cryopreserved PBMCs/vial/donor for 10 donors. The cryopreserved PBMCs are tested for sterility and serology, cell activation, and are pre-tested for performance in a T-cell proliferation assay and a DC:T cell stimulation assay. Data concerning demographics, previous clinical history and the hematological analysis pre-/post-thawing is available on request.
Following panels are available:
This panel contains 10 different donors assembled in order to cover the most frequent HLA class I alleles present in the Caucasian population and often contains some of the less frequent alleles. ImmunoSafe™-HLA1 panels are available as off-the shelf products for next-day delivery. In case the panel is not in stock, lead time can be up to 3 weeks. To inquire about the lead time for specific panel compositions, please contact our sales team.
This panel contains 10 different donors assembled in order to cover the most frequent HLA class II alleles present in the Caucasian population and often contains some of the less frequent alleles. ImmunoSafe™-HLA2 panels are available as off-the shelf products for next-day delivery. In case the panel is not in stock, lead time can be up to 3 weeks. To inquire about the lead time for specific panel compositions, please contact our sales team.
Learn more about our immunogenicity and immunotoxicity offerings
Matched plasma and serum
Matched plasma and serum are available on request from donors included into the ImmunoSafe™ panels in 1ml aliquots. For custom panels and new productions, lead time can be up to 3 weeks. To inquire about the lead time for specific panel compositions or for different aliquots format, please contact us.


