Immunogenicity-Immunotoxicity
Immunogenicity and Immunotoxicity
Immunogenicity and immunotoxicity constitute potential challenges to be considered in the development of new Biotherapeutics, Vaccines, Cell and Gene Therapies, and are therefore areas of intense study and discussion from both regulators and the scientific community.
Immunogenicity refers to the ability of a substance to stimulate an immune response; immunotoxicity, on the other hand, refers to the ability of a substance to cause adverse effects from the immune system or other systems as a result of a dysfunction o the immune system.
What is the role of Immunogenicity and Immunotoxicity in the development of novel protein therapeutics?
The evaluation of the immunogenicity and immunotoxicity risk of novel protein therapeutics is pivotal for guaranteeing their safety and efficacy, and it is subject to several challenges including variations in immunogenic response between different populations, the change in the immunogenicity of vaccines with age, and the general unsuitability of animal models in predicting immune responses in humans.
While immunotoxicity is usually an unwanted effect of any therapeutic approach, immunogenicity may be a desired (wanted) or undesired (unwanted) effect in novel protein therapeutics:
- Wanted Immunogenicity is a central aspect of vaccine efficacy, as the injection of the desired antigen stimulates an immune response against it, thus creating a protection against the active pathogen
- Unwanted immunogenicity constitutes the undesired immune response against protein therapeutics, often resulting in the production of anti-drug-antibodies (ADAs) with inactivating or neutralizing properties against the therapeutic agent.
Because of the potential unwanted effects of immunogenicity, regulators expect that developers of non-vaccine biological therapeutics employ validated immunogenicity assays to detect and characterize the risk of ADAs formation in the clinical development phases. During preclinical immunogenicity and immunotoxicity assessment, an early characterization of the innate and adaptive immune response against novel protein therapeutics can help de-risking the development pipeline and contribute in gaining useful insights in the mechanism of action or cues for patient segmentation in the clinical development phases.
What are the mechanisms of Immunogenicity?
Immunogenicity is a mechanism that can involve both cells of the innate immune system such as monocytes, macrophages, NK cells, mast cells and neutrophils and and cells of the adaptive immune system such as macrophages, denditic cells, T-Helper cells, T-Cytotoxic cells, T-Regulatory cells and B-Cells. The HLA profile of an individal constitutes an important contributing factor to the immune response, and it is therefore necessary to study cells from donors presenting an HLA profile representative of the target population in order to accurately set up immunogenicity assay
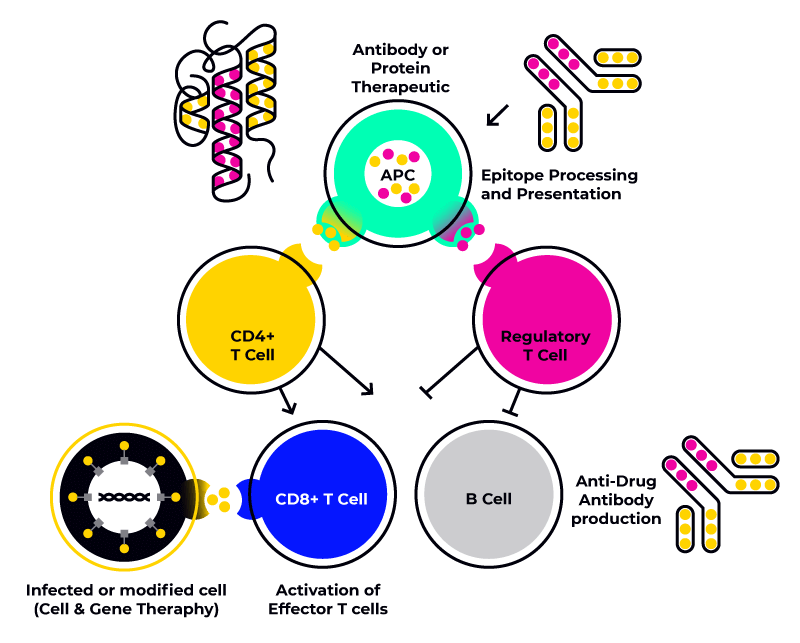
Immunogenicity in biotherapeutics
It is precisely this second, HLA class II-mediated mechanism, that leads to the formation of Anti-Drug Antibodies (ADAs), which leads to reduced efficacy of biotherapeutics as well as other adverse events, including hypersensitivity reactions and increased clearance of the therapeutic product.
Since TCRs recognize the HLA-epitope complex, it is possible to understand why specific HLA alleles have been identified as playing a role in the development of ADAs in multiple sclerosis and rheumatoid arthritis patients. For this reason it is important to screen biotherapeutics with a representative sample of immune cells (e.g. PBMCs) presenting the most common HLA class II alleles or haplotypes (group of alleles inherited together from a single parent) expressed by the target population.
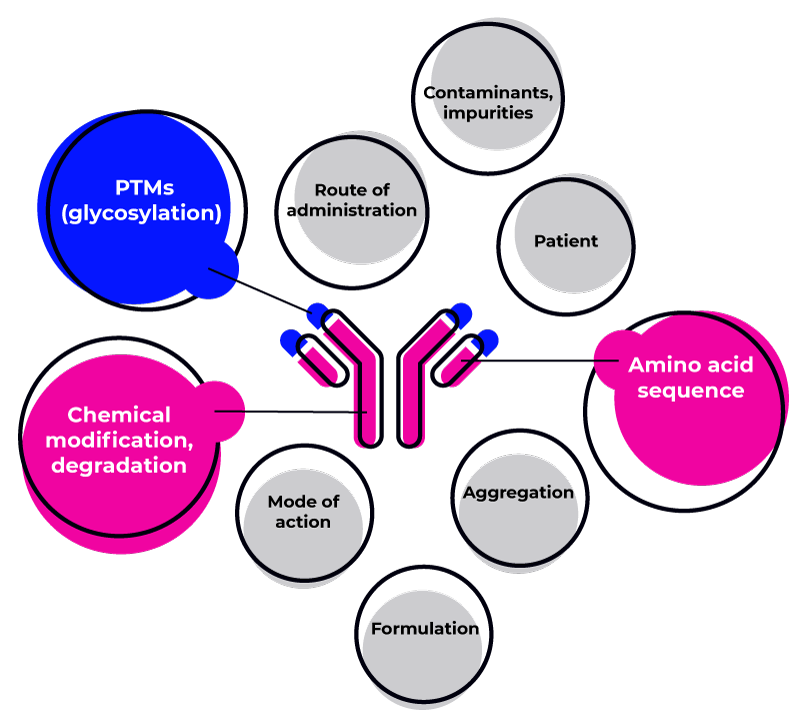
Common causes of immunogenicity include:
-
The HLA alleles of the patient
-
The amino acidic structure of the biotherapeutic
-
Possible aggregation of the biotherapeutic
-
Chemical modification or degradation
-
The route of administration
-
Possible previous diseases of the patient
-
Impurities and contaminations
-
Formulation and Dose
-
The mode of action (e.g., intra- or extracellular target)
-
Post-translational Modifications (PTMs) such as glycosylation and PEG-ilation
-
The presence of animal-derived components
-
The presence of animal-derived sequences in the biotherapeutic structure
The assessment of immunogenicity of a biotherapeutic can be performed in the preclinical development phases by performing an in silico analysis of the epitopes in a biotherapeutic, a cell-based screening of the selected epitopes on immune cells covering the most common HLA Class II, followed by in vitro immunogenicity assays predicting antibody binding and neutralizing responses. During clinical development, ADAs can be confirmed through detection in validated assays in blood samples from patients.
Immunogenicity in Cell and Gene Therapies and Vaccines
While HLA Class II-mediated adaptive immune response plays a major role in the development of immunogenicity against biotherapeutics (unwanted immunogenicity) and vaccines (wanted immunogenicity), HLA Class I-mediated immune response plays a major role in the development of Cell Therapies, Gene Therapies and Vaccines.
Cell Therapies are applications in which cells are expanded and reapplied to the damaged tissue. In case of allogenic cell therapies, it is important to consider that if the donor presents relevant polymorphisms in any of the endogenous proteins or expresses different HLA alleles, the new epitopes will be presented on HLA class I and the cells will be eliminated by either NK cells or CD8+ T-Cytotoxic cells.
Gene Therapies are applications in which cells are genetically modified either in vivo or ex-vivo. Similarly to ATMPs in which genetic manipulation on autologous or allogenic cells leads to introduction of new sequences and/or introduction of new epitopes, the newly formed epitopes will be presented by HLA class I and potentially eliminated by NK cells or CD8+ T-Cytotoxic cells.
Although the response to vaccines relies for a large part on the adaptive immune response through HLA Class II for the generation of neutralizing antibodies, the activation of CD8+ T-Cytotoxic cells is responsible for the elimination of infected cells in case of viruses and other intracellular pathogens, thus stopping the infection. mRNA vaccines have been proven to be an essential way to fight the recent COVID-19 pandemics and have established themselves as quick to develop vaccines against several pathogens. As their efficacy depends on the expression of pathogen-derived antigens in the recipient’s cells, monitoring the activation of HLA class I-mediated immune response is pivotal to ensure sufficient protection against the pathogen while avoiding toxicity at the site of injection due to the CD8+ T-Cytotoxic response against vaccine antigen-expressing cells.
It is therefore important to assess the immunogenicity of cell and gene therapies and vaccines by testing them against immune cells representing the most common HLA class I expressed in the target population.
Which assays are used in Immunogenicity testing?
The development of biotherapeutics and biosimilar biologics, Cell and Gene Therapies and Vaccines requires an extensive analysis of the immunogenicity risk of the drug substance as well of the drug product. The assessment of the immunogenicity risk is based on a wide range of assays that help you understanding the occurrence and causes of immonugenicity in your therapeutic approach and optimizing the right strategy.
Common assays for pre-clinical evaluation of the immunogenicity include:
-
In silico Epitope analysis
-
In vitro HLA Class I and Class II binding assays
-
Cell-based immunogenicity assays including T-cell proliferation assays, DC:T-Cell stimulation and proliferation assays and 2D-3D migration and activation assays in compley models
-
In vivo immunogenicity assessment with humanized mice models
-
Ex vivo assays with plasma or whole blood for the detection of ADAs
What are the mechanisms of Immunotoxicity?
Immunotoxicity consists in the ability of a substance to impair the humoral or cellular immune response, leading to immunosuppression or causing unnecessary tissue damage (such as in the cases of hypersensitivity, autoimmunity or chronic inflammation). Immunotoxicity can be divided in on-target and off-target immunotoxicity.
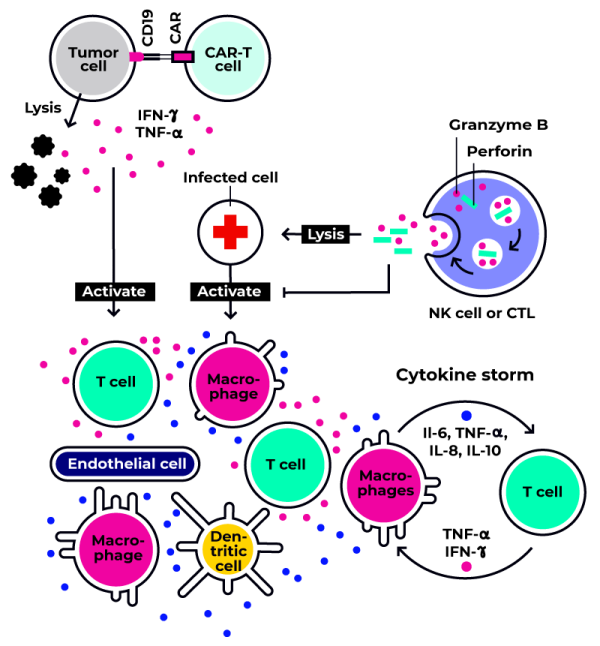
Immunomodulatory therapies as well as immune-based ATMPs such as CAR-T Cell Therapies are known to potentially induce CRS as a consequence of cell damage on- and off-target. It is therefore important to monitor the possible occurence of immunotoxic effects in order to implement appropriate strategies during early development.
On-target immunotoxicity is often induced by immunomodulatory monoclonal antibodies (mAb), and can result from exaggerated or prolonged activity of the mAb binding to the desired target antigen or the desired target cell/mediator. This can lead to the modulation of a target with multiple immune functions (including functions with no therapeutic benefit) and lead to adverse events.
Off-target immunotoxicity is often induced by mAb targeting epitopes presented by or a target expressed on non-immune cells or other cells not intended as a therapeutic target.
The cytokine release syndrome (CRS) constitutes one of the most severe consequence of immunotoxicity, whith possible lethal effects. In CRS, a cytokine storm is initiated as a consequence of a normal activation of the immune system against a pathogen or a foreign substance. During this reaction, the activated macrophages and dendritic cells realease cytokines to recruit and activate the lymphocytes and initiate the inflammation process. The successful elimination of the target/infected cells leads to the release of further inlammatory signals and induces damages to the lymphocytes. The damaged lymphocytes release further inflammatory cytokines and increase the intensity of the immune reaction to the point that the inflammatory response starts damaging the healthy tissues, leading to important functional damages.
What assays are used in Immunotoxicity testing?
Immunotoxicity constitutes one of the main considerations during lead selection and further optimization, and an early evaluation of on- and off-target toxicity is of pivotal importance for de-risking the development process. In order to study the immunotoxicity of a therapeutic strategy, it is important to understand its mechanism of action and what is the clinically acceptable risk/benefit ratio to the patient.
Common assays to evaluate immunotoxicity include:
-
In silico Epitope analysis
-
Cell-based off-target toxicity evaluation with primary cells from non-target tissues
-
Cell-based on-target toxicity evaluation of CRS
-
In vivo studies (depending on the pharmacology)
-
Ex vivo assays with plasma or whole blood for the detection of ADAs
Immunogenicity and Immunotoxicity panels
BIOMEX’s Immunogenicity and Immunotoxicity panels are designed to support the in-house safety testing of Large Molecules, Cell Therapies and Gene Therapies during the preclinical and clinical phases. The panels are assembled in a configuration suitable to reproduce the whole range of the most frequent HLA class I or class II alleles in the Caucasian population and are suitable for the the first screening assays. Panels to perform more specific assays with particular alleles can be assembled upon request.
The Immunogenicity and Immunotoxicity panels include each 100 vials, corresponding to 10 vials à 20 Mio viable cryopreserved PBMCs/vial/donor for 10 donors. The cryopreserved PBMCs are tested for sterility and serology, cell activation, and are pre-tested for performance in a T-cell proliferation assay and a DC:T cell stimulation assay. Data concerning demographics, previous clinical history and the hematological analysis pre-/post-thawing is available on request.
Following panels are available:
This panel contains 10 different donors assembled in order to cover the most frequent HLA class I alleles present in the Caucasian population and often contains some of the less frequent alleles. ImmunoSafe™-HLA1 panels are available as off-the shelf products for next-day delivery. In case the panel is not in stock, lead time can be up to 3 weeks.
This panel contains 10 different donors assembled in order to cover the most frequent HLA class II alleles present in the Caucasian population and often contains some of the less frequent alleles. ImmunoSafe™-HLA2 panels are available as off-the shelf products for next-day delivery. In case the panel is not in stock, lead time can be up to 3 weeks.
To inquire about the lead time for specific panel compositions, contact us.
Matched plasma and serum
Matched plasma and serum are available on request from donors included into the ImmunoSafe™ panels in 1ml aliquots. For custom panels and new productions, lead time can be up to 3 weeks. To inquire about the lead time for specific panel compositions or for different aliquots format.
Immunogenicity and Immunotoxicity services
Our Immunogenicity and Immunotoxicity services are designed to provide you with comprehensive information on the risk profile of your therapeutic candidate and enable you to further refine, humanize or engineer your biotherpeutic and evaluate its potency and efficacy.
Our strategy consists of in silico and in vitro assays to identify immunogenic epitopes, study the interaction of immune cells and identify suitable candidates.
BIOMEX has developed a range of in vitro assays including:
-
T-Cell activation and proliferation
-
Innate immune cells activation and proliferation
-
DC:T-cell stimulation assays
-
Cytokine Storm assay
If you are interested in developing new assays or in studying particular mechanisms of actions, our Service team will partner will you to develop the optimal solution.
For more information on how to de-risk the development of your therapeutic:


