FAQ Diagnostics
Frequently Asked Questions:
BIOMEX’s Performance Evaluation Studies
We are your experts in performance evaluation studies for in vitro diagnostics and assay validation. Here you will find answers to frequently asked questions on these topics. Please do not hesitate and contact us directly with any further requests.
1. Background and Expertise
All main facilities are conveniently based in Heidelberg, Germany, one of Europe’s main biological research hubs. Heidelberg is only a one-hour drive or train ride from Germany’s largest airport in Frankfurt am Main.
Among other things, the following benefits set us apart from other CROs and give us the ability to conduct your performance evaluation study with maximum flexibility and quality:
- Sample collection in our own donation centers – prospective and exactly tailored to your specific requirements
- Europe’s largest commercial biospecimen inventory
- 100% value chain in-house, including expert regulatory support, study design, ethics approval, testing in our own labs, material handling and external site management
We started to deliver performance evaluation studies for in vitro diagnostics in 2017. Since then, we have supported numerous manufacturers by conducting hundreds of performance evaluation studies.
We collect 90% of samples through our own donation centers in Heidelberg, Munich and Cameroon (Africa). Our inventory holds over 600,000 biospecimens for you: Plasma, Serum, Swabs and Tissue. For more information, please visit our Sampleshop. In addition, we constantly obtain residual samples via our worldwide network and carry out prospective sample collections for you upon request.
We operate laboratories that allow us to work with samples of biosafety level 3. These include, for example, HIV, HCV, or SARS-CoV-2.
In addition to standard laboratory methods, we offer various tests such as PCR, clinical chemistry, CLIA/CMIA, POCT and more. Our equipment includes:
| Company | Instrument |
|---|---|
| Roche | Cobas e411 |
| Roche | Cobas c501 |
| Roche | Cobas 5800 |
| Abbott | Architect i1000SR |
| Diasorin | Liason XL |
| BioMérieux | MiniVidas |
| ThermoScientific | Quantstudio 5 |
| Bio-Rad | CFX96 (x2) |
Of course. We maintain an “open door policy” and are happy to show you our infrastructure on site.
2. Performance evaluation studies
In addition to clinical and analytical performance studies, you can work with us to evaluate near-patient testing (NPT) / Point-of-care testing (POCT), self-tests for laypersons, and CLIA assays:
| Study Types | How we work |
|---|---|
| Clinical performance evaluation studies | We operate our own donation centers. This enables the sourcing of positive and negative samples for the evaluation of diagnostic sensitivity and specificity, as well as for the majority of common analytes. |
| Analytical performance evaluation studies | We perform full validation studies for IVDs (qualitative or quantitative) according to the relevant CLSI guidelines. This includes analytical sensitivity (limit of detection, limit of quantification), analytical specificity, stability of the assay or sample, trueness, precision, accuracy, linearity, measuring range, and cut-off value. |
| Laymen studies, Near-patient testing / Point-of-care testing | Through our own test centers, we can effectively recruit lay people to participate in usability/laymen studies or NPT/POCT studies. |
| Studies for the validation of CLIA-assays | Our infrastructure is capable of performing tests with class D IVDs (e.g., HIV) as well as class C and B IVDs in accordance with the EU common specifications. We have dedicated facilities and instruments for reference testing to routinely perform these validation studies in-house. |
Yes, in contrast to many other CROs, we offer a full service that includes study design, all related consultations by our experts in the field, sourcing and preparation of all necessary samples, in-house testing in our own laboratories, and data analysis and preparation of the final report.
Please contact our sales team at sales@biomex.de. Our experts will guide you through our standardized inquiry process.
The study duration depends on several factors, such as the type of the study and the sample, the sample size, the time required for collection (for example, incidences and seasonal events have a large impact on prospective sample collection), the time required for IRB approval, and the time to set up your study. Thanks to our many years of experience, we can usually provide an estimate in advance.
We generally do not conduct research on scientific validity unless it is commissioned as part of a larger package that includes scientific validity, analytical performance, and clinical performance.
Yes, in addition to our large biobank of retrospectively collected samples, we have facilities and infrastructure to collect fresh biospecimens for your study. This includes recruitment of participants and obtaining approvals from the institutional review board (IRB).
Depending on study requirements, we may collect the usual clinical information of each donor, e.g., age, sex, time/date of sample collection, initial diagnosis, symptoms and date of symptoms onset, information regarding contact with infected persons and/or vaccination status, ICD code (provided this does not interfere with sample anonymization requirements). Prospective collection of non-anonymized samples is also possible with the involvement of clinical cooperation partners after submission of a positive ethical vote.
We are a strong partner for all IVDR (European Union), TGA (Australia), NMPA (China) and WHO guideline applications. We do not offer studies (especially clinical) for FDA (USA) applications.
We do not specifically advise or assist in the selection of the Notified Body, but will be happy to refer you to one of our trusted partners. Please inquire at sales@biomex.de.
We do not act as a legal representative but will be happy to refer you to a trusted partner of ours. Please inquire at sales@biomex.de.
3. Quality and ethics
Our business activities are determined by the wishes of our customers and the legal requirements, and are guided by responsibility towards society and the environment. We produce and supply high-quality products at the cutting edge of science and technology and provide a high level of service. Our premise is reliability and customer-oriented action. We strive to avoid errors instead of subsequently correcting them. The requirements of our quality management system are always met, and we continuously improve its effectiveness. If you want to learn more about our corporate philosophy, please visit our company pages.
Our quality management system is accredited according to ISO 9001. All studies are performed in accordance with ISO 20916:2019 and ISO 14155. Furthermore, we are currently in the process of obtaining ISO 17025 certification for our laboratories.
Yes, for both invasive and non-invasive clinical sample collections.
For non-invasive testing, we have ethical approval for participants 14 years of age and older. For invasive testing, we have ethical approval for participants 18 years of age and older. If ethical approvals are required for participants younger than the available IRB, these can be applied for.
In addition to standard laboratory methods, we offer various tests such as PCR, clinical chemistry, CLIA/CMIA, POCT and more. Our equipment includes:
| Company | Instrument |
|---|---|
| Roche | Cobas e411 |
| Roche | Cobas c501 |
| Roche | Cobas 5800 |
| Abbott | Architect i1000SR |
| Diasorin | Liason XL |
| BioMérieux | MiniVidas |
| ThermoScientific | Quantstudio 5 |
| Bio-Rad | CFX96 (x2) |
Among other things, the following benefits set us apart from other CROs and give us the ability to conduct your performance evaluation study with maximum flexibility and quality:
- Sample collection in our own donation centers – prospective and exactly tailored to your specific requirements
- Europe’s largest commercial biospecimen inventory
- 100% value chain in-house, including expert regulatory support, study design, ethics approval, testing in our own labs, material handling and external site management
Of course. We maintain an “open door policy” and are happy to show you our infrastructure on site.
4. In Vitro Diagnostics Regulation (IVDR)
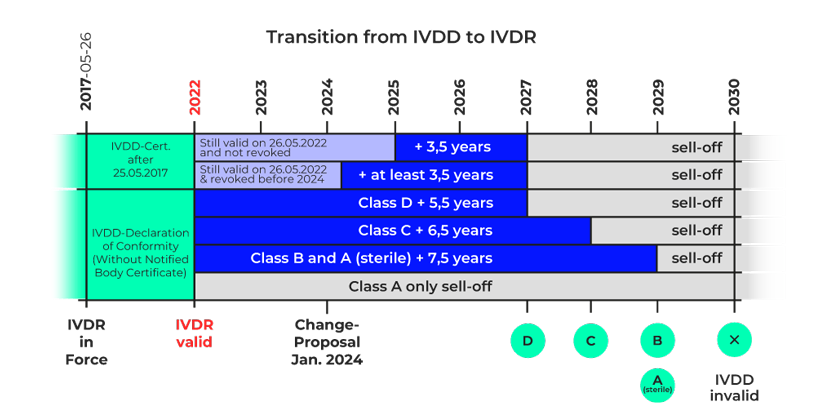
The European In Vitro Diagnostics Regulation (IVDR) came into force in May 2017, replacing the previous In Vitro Diagnostics Directive (IVDD) in May 2022 after a five-year transition period. Until May 2027, the specifications will gradually become valid for the individual product risk classes.
Analytical performance refers to the ability to correctly detect or measure a specific analyte (IVDR, Article 2 (40)).
Clinical performance describes the ability to provide results that correlate with a specific clinical condition or physiological or pathological process or state (IVDR, Article 2 (41)).
After market introduction, manufacturers should proactively collect and evaluate performance and scientific data resulting from the use of the device to ensure safety, performance and scientific validity throughout the lifetime of the device and to identify any risks (IVDR, Annex XIII, Part B, 4).
Your Experts for Diagnostic Studies
You want to bring your assay to market as quickly as possible? We can help you with this – with our years of experience in diagnostic assay validation, broad regulatory expertise, large in-house biospecimens inventory, and our own donations centers.


