More than 900 sequential bleeds have been collected by BIOMEX since 9th of April from over 150 recovered COVID-19 patients – i.e. 2 bleeds per week.
The units were tested with CLIA- (IgM u. IgG) as well as with ELISA-tests (IgM, IgG & IgA).
As a first observation, we have seen that IgG levels differ significantly among donors and not all donors develop IgM Abs.
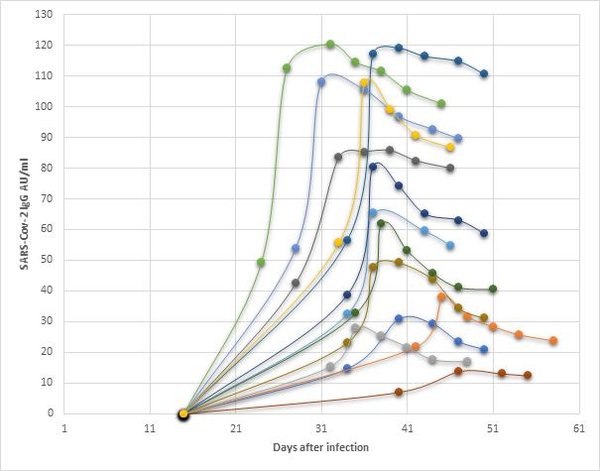
The following graph shows the IgG values of a small selection of donors tested with a CLIA system. One of the main challenges for assay developers is the determination of an appropriate cut off value to detect all IgG responses including lower levels. Once the cut-off is established, validation of the assay with a population of clinically confirmed positive samples is necessary.
BIOMEX offers IvD manufacturers assistance in finding a valid cut-off level and in performing a clinical validation with a variety of clinically confirmed positive and negative samples required for the diagnostic sensitivity and specificity.
Please contact sales@biomex.de or +49 6221 – 894 669-43 to get feedback regarding your specific needs.